This audio is automatically generated. feedback.
In a disastrous decade, pharmaceutical companies GSK and AstraZeneca To Novartis and Pfizer Clinical failure, investor risk, and a lack of scientific justification led many pharmaceutical companies to shut down their neuroscience drug development programs, but in recent years there has been evidence of a brain resurgence across the industry.
Behind the shift is a group of smaller biotech companies that have been working hard to pursue central nervous system research even as big pharmaceutical companies retreated. Now, as the industry’s larger players renew their interest in the field, these companies are reaping the rewards of a resurgence of collaborations and value deals.
Some of the biggest recent fights in the CNS field have been with Bristol-Myers Squibb $14 billion purchase Karuna Therapeutics to announce at the end of 2023, Pfizer $11.6 billion acquisition AbbVie also announced plans to acquire Biohaven in 2022. $8.7 billion These deals, led by its acquisition of Cerevel late last year and centered around candidates for schizophrenia, migraine and Parkinson’s disease, marked a turning point in the value that big pharma placed on neuroscience research.
“There’s a renewed interest in central nervous system diseases and for those who have been in the field for a long time, it’s good to see investment coming back.”

Gene Kinney
CEO of Procena Biosciences
Clinical and regulatory successes are also helping the CNS comeback. For example, despite the challenges Biogen and Eisai faced with their Alzheimer’s drug Aduherm, the turmoil paved the way for the companies’ follow-up, Lukembi, to hit store shelves with greater impact, and Eli Lilly’s next product, Potential approval of donanemab It’s a testament to how quickly that situation is changing.
As new treatments for Alzheimer’s disease, depression and other brain disorders become better defined and investment risks decrease, the CNS development field is experiencing a resurgence as a result of scientific advances and modern approaches.
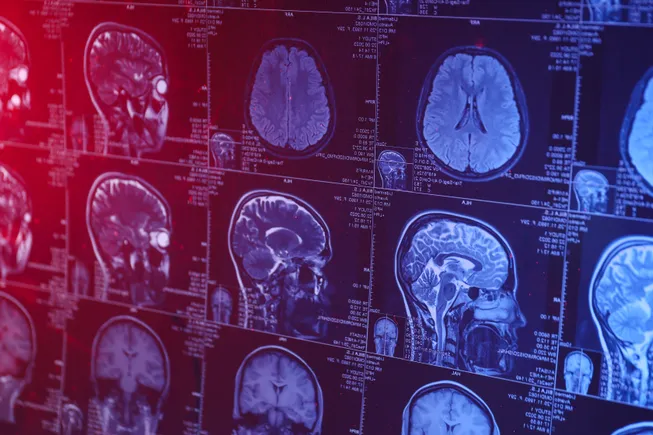
Protena’s Brain Treasure

Gene Kinney, CEO of Prothena Biosciences
Used with permission from Prothena
Beyond pure acquisitions in the CNS space, pharmaceutical companies are also looking to partner with smaller companies as they expand into neuroscience. Prothena Biosciences, which specializes in neurodegenerative protein dysregulation, is one biotech company in the process of partnering with a large pharmaceutical company.
One of the early collaborations symbolizing the new wave of interest in CNS was a $2 billion milestone deal between Celgene and Prothena in 2018. Today, after Celgene was acquired by Bristol-Myers, Prothena continues to work with the pharmaceutical giant to develop new CNS drug candidates.
Recently, Bristol-Myers Exclusive License Last month, the company approved the acquisition of Prosena Inc.’s neurodegenerative drug PRX019. While the companies have not disclosed a formal target price for the candidate, the deal will net the biotech $80 million, and Prosena plans to begin early-stage testing by the end of the year.
“There’s been a resurgence of interest in central nervous system diseases, and for those of us who have been in the field for a long time, it’s good to see investment coming back into an area where investment had previously shifted away,” said Gene Kinney, CEO of Prosena. “This is fantastic, given the burden of neurodegenerative disease in our ageing population. We’re seeing fantastic progress in clinical trials.”
Kinney cited reasons for the resurgence including the discovery and development of new biomarkers, more sophisticated patient selection processes, more sensitive and meaningful endpoints, and “an overall strengthened patient voice in clinical trial conduct and regulatory science.”
He also noted that improvements in AI will benefit scientific advances in understanding protein structure and in the early stages of research.
“There are some really exciting technologies out there that are opening up whole areas of science to explore questions that were previously unreachable,” Kinney said. “And with that come really exciting techniques for controlling proteins at the genetic level, making, aggregating and removing proteins inside and outside of cells.”
Prothena’s late-stage pipeline hopefuls include a Phase 3 AL amyloidosis candidate with results expected later this year or early 2025. The company also has prasinezumab, a Parkinson’s disease candidate in Phase 2 development with Roche, and Coramitsugu, an ATTR amyloidosis treatment in Phase 2 development with Novo Nordisk.
The biotech’s Alzheimer’s disease drug candidates include a tau-clearing therapy licensed to Bristol-Myers in mid-stage trials, an amyloid-clearing therapy in Phase 1 trials, and a potential amyloid-tau combination drug that is set to enter clinical trials.
As the science advances and pharmaceutical companies invest more in their neuroscience pipelines, the drug shortages of the past decade will likely be resolved and a wave of new drugs could hit the market in the coming years.
“The goal here is to translate science into therapies that help patients, and to do that as quickly as the data warrants,” Kinney said. “That’s key, and working with a group like Bristol-Myers, who has the scientific, clinical and commercial expertise, makes sense for us in terms of what they can bring to the program.”