This audio is automatically generated. feedback.
This summer has been full of biotech and pharmaceutical news. Termination of an employee Cuts to drug development programs are also being made, but those could slow as more deals are made.
In August, Vil Biotechnology, Unicorn and Arbutus The company joins a growing number of companies that are cutting jobs, and while cuts will likely continue in the short term, some industry watchers expect them to taper off by the end of the year as companies seek to replenish their dwindling talent pipelines with increased M&A activity.
Blockbuster GLP-1 Obesity medication “Emerging markets are gaining momentum, but companies need to rethink outdated business models that are holding them back,” said Michael Abrams, managing partner at health-care consultancy Numeroff & Associates.
A turbulent market
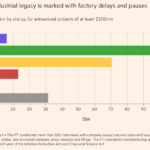
The pharmaceutical industry is on the brink of exponential growth and is cutting jobs to prepare for declining revenues. Patent CliffSome have been hit hard, with blockbuster drugs such as AbbVie’s Humira, Merck’s Keytruda and Bristol-Myers Squibb’s Opdivo having lost or soon will lose exclusivity, leaving them vulnerable to an array of biosimilars.
Abrams said the industry is trying to attract nervous investors. A pullback in investment since the pandemic has left 31% of biotech companies at risk of running out of funding within a year, according to a research firm. EY Industry ReportsMany investors are shying away from riskier drug development opportunities and putting their money into safer bets. Doctor Group Practicehe said.

Michael Abrams, Managing Partner, Numerof & Associates
Used with permission from Numerof & Associates
“Pharmaceutical companies need to pay dividends and, ideally, get some valuation so they don’t move capital elsewhere,” Abrams said.
Pressure from Inflation Control Law Reining in high drug prices through Medicare is bad for the industry. The Federal Trade Commission becomes more aggressive Abrams also said it’s making some business leaders nervous.
Fears about the IRA’s impact remain widespread, but some executives say they are hesitant to accept IRA-negotiated discounts. Not so steep That’s as initially feared, but some industry leaders are still wary of the FTC and worry that the regulations could stifle pharmaceutical innovation.
“If the FTC challenges the acquisition, it could drag out the entire process and potentially lead investors to go elsewhere,” Abrams said.
Favorable signs
Abrams said the future could be bright for small and mid-sized biotech companies that advance drug development if the Federal Reserve delivers its long-awaited interest rate cuts this fall.
“You’d expect that with all the resources at their disposal, big pharma would be responsible for the vast majority of drug approvals,” Abrams said, “but that’s not the case.”
“If the FTC were to challenge the acquisition, it could drag out the entire process and potentially lead to investors moving elsewhere.”

Michael Abrams
Managing Partner, Numerof & Associates
He said smaller organizations account for more than half of new prescription drug approvals, but the road for these companies has been tougher recently because of high interest rates, although their prospects would likely improve if the Fed were to cut rates.
“If interest rates start to come down, I think it will be a positive for smaller organizations that still need capital because it will be easier to get financing because they will no longer be competing with other lower-risk investment options and the chances of recovery will be just as good,” he said. “That means we will see other players in the acquisition game and other funders.”
Oncology, rare diseases and immunology are likely to remain hot targets for investors, with buying drugs from smaller start-ups offering advantages over developing them in-house.
“Big pharma is removing high-risk, low-probability products from their R&D portfolios and replacing them with acquisitions that essentially have greater potential than the products they’re cutting,” Abrams said.
M&A can also give companies a foothold in emerging markets. See AbbVie’s recent acquisition Acquisition Cerebelle Therapeutics’ next-generation schizophrenia treatment emracidine is a BMS Potential blockbuster KarXTIt could be approved this fall.
And in terms of capital to make these deals, big pharma has it: Many have piles of cash amassed from successful products over the past decade, Abrams said. By 2023, companies will have roughly $1.37 Trillion in Deployable Capacity.
“You’re going to see a lot of M&A activity through the rest of this year and into next year,” Abrams said.
Variables to consider
Also factoring in the ongoing pharmaceutical industry conversation is the upcoming election and how it will affect Medicare drug price negotiations mandated by the IRA. While the industry is still fighting lawsuits in court against the IRA, recent polls indicate that the election results are unlikely to lead to a repeal of the law due to public support for reducing high drug costs. Evaluate the report.
As companies change to adapt to new realities, Abrams said, they also need to rethink traditional marketing strategies in a changing marketplace. Drug purchasing decisions are increasingly being made not just by individual physicians, but by representatives of health systems eager to choose options that deliver value and strong clinical outcomes.
“Most manufacturers are looking to restructure their sales approach and their sales organizations to keep up with these changes that are making them less and less efficient on the sales side. They have to find new ways to reach these customers,” Abrams said. “The old ways are no longer effective.”
Abrams said the pharmaceutical industry has experienced setbacks and adapted to change, but it seems poised for a breakthrough.
“We are entering a period of a lot of M&A activity and the overall business development sector will continue to boom,” he said.