It is estimated that more than 18 million cases of cancer occurred worldwide in 2020.
Leuko, founded by researchers at the Massachusetts Institute of Technology (MIT), has developed a home white blood cell monitor that can help doctors noninvasively monitor the health of cancer patients undergoing chemotherapy.
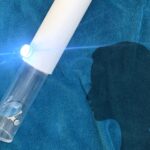
The PointCheck device provides an easy-to-use way for physicians to track immune health and optimize patients’ cancer treatment.
According to Cancer Research UK, it is estimated that more than 18 million cases of cancer occurred worldwide in 2020.
Chemotherapy works to destroy cancer cells, but destroying cancer cells can also destroy a patient’s immune cells, resulting in a weakened immune system and increased risk of infection for tens of thousands of cancer patients each year.
To strike a balance between giving enough chemotherapy to destroy the cancer while making sure a patient’s white blood cell count doesn’t drop too much, Leuko’s PointCheck device uses light to see through the skin above the fingernail and uses artificial intelligence to analyze and detect when white blood cells reach low levels, without the need for a blood test.
First developed in 2015, a study of 44 patients conducted by MIT researchers in 2019 demonstrated that PointCheck could detect when white blood cell levels fell below a critical threshold with minimal false positives.
A larger study of 154 patients demonstrated that the PointCheck device can be easily used at home by unsupervised patients to provide immune information to physicians.
The research team also believes that the device could potentially be used in the future to monitor other biomarkers in the blood for other diseases.
“Other patient populations include multiple sclerosis, autoimmune diseases, organ transplant patients and emergency room patients,” said Carlos Castro Gonzalez, co-founder and CEO of Royco.
Ian Butterworth, co-founder and chief technology officer at Leuko, commented: “We hope this will bring about a clear improvement in the way outpatients are monitored and cared for.”
The company plans to conduct a pivotal study to prepare for U.S. Food and Drug Administration approval later this year.