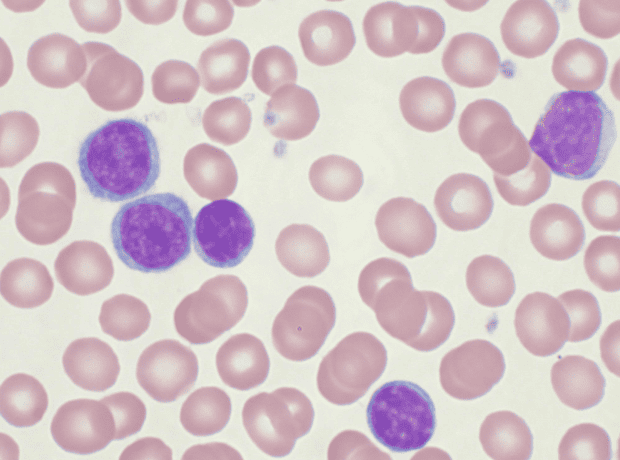
This subtype accounts for 20% of all T-cell leukemias and is a rare blood cancer in which too many abnormal T-cells are produced.
OneChain Immunotherapeutics (OCI), a biotechnology company specializing in the development of CAR T-cell therapies for oncological diseases, has announced that it has administered OC-1 to the first cortical T-cell acute lymphoblastic leukemia (coT-ALL) patients in the CARxALL clinical trial. announced that it had been administered. CAR T therapy.
The trial will be conducted at the Clinic Hospital of Barcelona and the Sant Joan de Deu Hospital and is open to both pediatric and adult patients from all over the world.
T-cell leukemia is a rare form of blood cancer that occurs when the bone marrow produces too many abnormal T cells, a type of white blood cell that protects the body from infection.
The subtype coT-ALL accounts for 20% of T-cell leukemias and is characterized by a poor prognosis for patients who do not respond to existing treatments.
CAR T therapy is a type of immunotherapy in which immune cells, known as T cells, are genetically modified to enhance and target their ability to recognize and attack cancer cells.
This study aimed to evaluate the safety and tolerability of OC-1 for the first time in humans in patients with coT-ALL who had previously received several treatments without response.
The treatment, which has already received orphan drug designation from the European Medicines Agency and the U.S. Food and Drug Administration, works by targeting CD1a, a protein found only in tumor cells in patients with coT-ALL, making it less competitive. may reduce the risk of severe immunosuppression associated with CAR T. Treatment.
“This treatment brings hope to patients who have exhausted all available options,” said Nuria Martinez, principal investigator at the hospital clinic.
Dr. Stefanos Theoharis, Chief Executive Officer of OCI, commented: “This is an important milestone and a very proud moment for OCI and all our partners.”
The biotechnology has four other projects aimed at treating patients with T-ALL, B-cell ALL, glioblastoma multiforme, and a CAR for allogeneic application known as OC-3. A Vδ1 gamma delta T cell platform is also under development. Ready-to-use treatments at low cost.