Yves here. Most of us know about anchoring in the concept of pricing and creating quantitive expectations in bidding and negotiating contexts, and in bigger frames like scientific paradigms, but here we see how it operates in smaller but still very important contexts. KLG today focuses on the question of concept anchoring and how it has played out to the disadvantage of research on mental disorders.
His intro:
Concept anchoring can set scientists and scholars in any field on the wrong path. Two prominent examples are considered here in an update of previous posts: (1) the “amyloid hypothesis” as an explanation of Alzheimer’s disease (AD) and (2) the “chemical imbalance hypothesis” as an explanation for depression. In the first case, more than 30 years of work dominated by the amyloid hypothesis has yielded scant advances in our understanding of AD. Although the hypothesis was reasonable at first, it is still not really known if amyloid plaques are cause, correlation, or effect of AD. It is also clear that research misconduct may have contributed to concept anchoring in AD. Regarding depression, the original hypothesis that serotonin deficits cause depression was reasonable based on a simple understanding of neurotransmission. But like the amyloid hypothesis for AD, the chemical imbalance hypothesis has not lead to much sustained progress. This is finally being recognized with new antidepressants such as ketamine that induce neuroplasticity in the brain and reverse the maladaptive responses to the world of those with depression.
By KLG, who has held research and academic positions in three US medical schools since 1995 and is currently Professor of Biochemistry and Associate Dean. He has performed and directed research on protein structure, function, and evolution; cell adhesion and motility; the mechanism of viral fusion proteins; and assembly of the vertebrate heart. He has served on national review panels of both public and private funding agencies, and his research and that of his students has been funded by the American Heart Association, American Cancer Society, and National Institutes of Health
The first post in this series two years ago was an analysis of Evidence-Based Medicine, which is commonly viewed as the “conscientious, explicit, and judicious use of current best evidence in the practice of medicine.” I have since begun making a distinction between Biomedical Science and Biomedicine. Biomedical Science is the “disinterested pursuit of useful knowledge, both for its own sake and for the improvement of human health and wellbeing.” The goal of Biomedical Science is to produce useful knowledge that approaches the truth and serves as the foundation for what comes next.
Biomedicine, on the other hand, is the public face of Evidence-Based Medicine (EBM), and, it should never be forgotten, often an appurtenance of Big Pharma and Big Medicine. Thus, EBM is often as much marketing as science, and we have not been well served by it, as illustrated in direct-to-consumer (sic) ads for prescription drugs seen on TV in the USA. Drugs like Ozempic have been all the rage lately. However, it is not entirely clear they are as benign in the long term as they are effective at inducing weight loss in the short term. And even in the short term, these drugs are a technical fix for a problem that should not exist. The role of Biomedicine in responses to COVID-19, which consisted of mRNA vaccines and little else in the first years of the continuing pandemic, is an ongoing story. A comprehensive comparison of the response to HIV/AIDS forty years ago and COVID-19 is likely to be useful.
Alzheimer’s disease (AD) in naturally a subject for Biomedical Science and even more so for Biomedicine. The disease is horrific, and every one of us of a certain age wonders on occasion if the inability to recall a word or a name is our first (recognized) symptom. After more than thirty years of well-funded research, we seem to be no nearer to understanding the causes of AD than we were when Alois Alzheimer described his first patient, Auguste D, who when asked to write her name replied, “Ich habe mich verloren – I’ve lost myself.” The question is: Why are we no closer to answers, if not a solution, to AD? The answer is likely to be that Biomedicine has gone all-in on the amyloid hypothesis as an explanation of AD.
This was covered previously here as a cautionary tale. The amyloid hypothesis is straightforward:
Amyloid precursor protein (APP) is a protein of unknown function found on the surface of cells in many tissues, including the central nervous system. Cleavage of APP by specific enzymes can produce the amyloidogenic (amyloid: aggregates of insoluble protein, found in many disease states) fragment that first forms soluble oligomers of a relative few A-beta peptides (i.e., smaller fragments of APP). These oligomers then aggregate into the insoluble A-beta plaques in AD brain tissue that were first observed over 100 years ago. It is thought that these aggregates lead to synaptic dysfunction and cell death in the brain.
But is AD really this simple? Perhaps, but whether amyloid plaques are cause, correlation, or effect has never been determined. A key paper (>1200 citations) supporting the conventional wisdom of AD was recently retracted, eighteen years after it was published, and two years after demonstration that the paper contained several “fatal flaws”:
The authors wish to retract this article. Concerns have been raised regarding figures in this article, including Fig. 2c and Supplementary Fig. 4, which show signs of excessive manipulation, including splicing, duplication and the use of an eraser tool. The data cannot be verified from the records. (1) We consider that the appropriate course of action is to retract the article.
Ming Teng Koh, Linda Kotilinek, Rakez Kayed, Charles G. Glabe, Michela Gallagher and Karen H. Ashe agree with the retraction. Sylvain Lesné disagrees with the retraction. Austin Yang has not responded to correspondence from the Editors about this retraction.
Progress of a sort, after a very long time in the world of scientific research. Sylvain Lesné is the author most responsible for the paper. Whether he is responsible for what has been called “scientific fraud” has been covered at length, and Dr. Lesné still has much explaining to do. But the much larger problem is the amyloid hypothesis foreclosed other research into AD, which could be caused by toxins and/or infectious agents such as herpes virus. Scientists who propose such research are not “members of the club” and their proposals are rarely funded. Their publications have been shunted aside into “second-tier” journals. And Biomedicine has been largely responsible for this, with the development of expensive monoclonal antibody therapies for the resolution of amyloid plaques in patients with AD receiving the most attention as therapeutics for AD. These antibodies, e.g., Aduhelm (Biogen), “work” but they neither prevent nor reverse AD. An early mouse model of AD showed that an antibody against amyloid completely eliminated plaques but did not alter the outcomes for the mice. The response to this was that mice are not a good model for AD, but maybe the plaque hypothesis is not a good hypothesis for AD. This is discussed here. Analogy with mRNA vaccines for COVID-19 comes to mind.
Alzheimer’s disease has become something of an object lesson of how not to do science. A scientist at CUNY has been recently indicted for fraud because he fabricated and falsified data in his research on AD. A criminal indictment for scientific fraud has been rare, but this may become more common when a good case can be made. Image manipulation is a common theme in the CUNY case and the University of Minnesota case of Sylvain Lesné. The former president of Stanford University came to grief over previous research on AD, but his downfall was “because he failed to adequately lead his labs,” perhaps due to pressure to produce desired results. The way we support and fund Biomedical Science must change, as noted in a recent article about the Lesné case and another at the University of Minnesota, but this seems to be a dream at the moment:
The studies are more than a decade old and superseded by other discoveries in their fields. But the retractions of the Alzheimer’s paper on Monday and the stem cell paper on June 17 are setbacks for an institution that is fighting to move up the U.S. rankings in academic reputation and federal research dollars.<
Both studies were published in the prestigious journal Nature and collectively have been cited nearly 7,000 times. Researchers worldwide were using these papers to support their work years after they had been disputed.
How much wasted effort is embodied in those 7,000 citations? How much did the University of Minnesota invest in the importance of this research, although the cases of Charles Lieber of Harvard (2) and Marc Tessier-Lavigne at Stanford show that similar behavior is widespread up and down the received scale of “academic excellence”?
More significantly, these cases are the result of how “anchoring” can set a field on the wrong path for a long time. An example outside of science is the Phillips Curve linking inflation with high levels of employment. The Phillips Curve is valid only for the years 1955-1970, which were the central years of the Great Compression. If surrounding years of the 20th century are included the correlation vanishes. Still, the Federal Reserve persists in its mistaken belief that the only response to inflation, no matter the cause, is to make working people pay in unemployment with attendant consequences that clients of the Federal Reserve never experience. The Laffer Curve showing the relationship between tax rates and government revenue is the homonymic champion in the category of “economic sciences.”
Anchoring in a different biomedical field may be lessening its grip, as shown in Beyond the serotonin deficit hypothesis: communicating a neuroplasticity framework of major depressive disorder, published in Molecular Psychiatry on 31 May 2024 (paywall). The accepted “chemical imbalance hypothesis” holds that depression arises from a deficit of serotonin, norepinephrine, and dopamine, with the corollary that drugs that restore normal levels of serotonin and other monoamines will alleviate depression. For the past 20 years this hypothesis has been considered incorrect due to a lack of evidence supporting it. But despite this lack of evidence “the serotonin deficit hypothesis has persisted in the public psyche, bolstered by pharmaceutical company advertisements for conventional antidepressants.” This is not to say that these classic antidepressants do not work. In many patients they outperform a placebo. Nevertheless, the oversimplifications of the chemical imbalance hypothesis and the overselling of these drugs has damaged the public’s faith in the Biomedical Science of depression, now generally called MDD – major depressive disorder.
The challenge of a new model of MDD is that “in must be simple enough to understand nut complex enough to be accurate, as well as adaptable enough to incorporate future research discoveries.” Basically, the chemical imbalance hypothesis was always too simple and too reductionist to account for MDD. But it did fit in with the spirit of the times. The triumphs of a thoroughly reductionist molecular biology from the early-1950s through the mid-1970s in explaining the molecular basis of genetics and gene regulation led to the extension of simple molecular models to most things biomedical. Very few dissenters were heard, and neuropsychiatry eventually triumphed: “Yes, we have a pill for that.” The biopsychosocial contributors to MDD were largely ignored (neuro).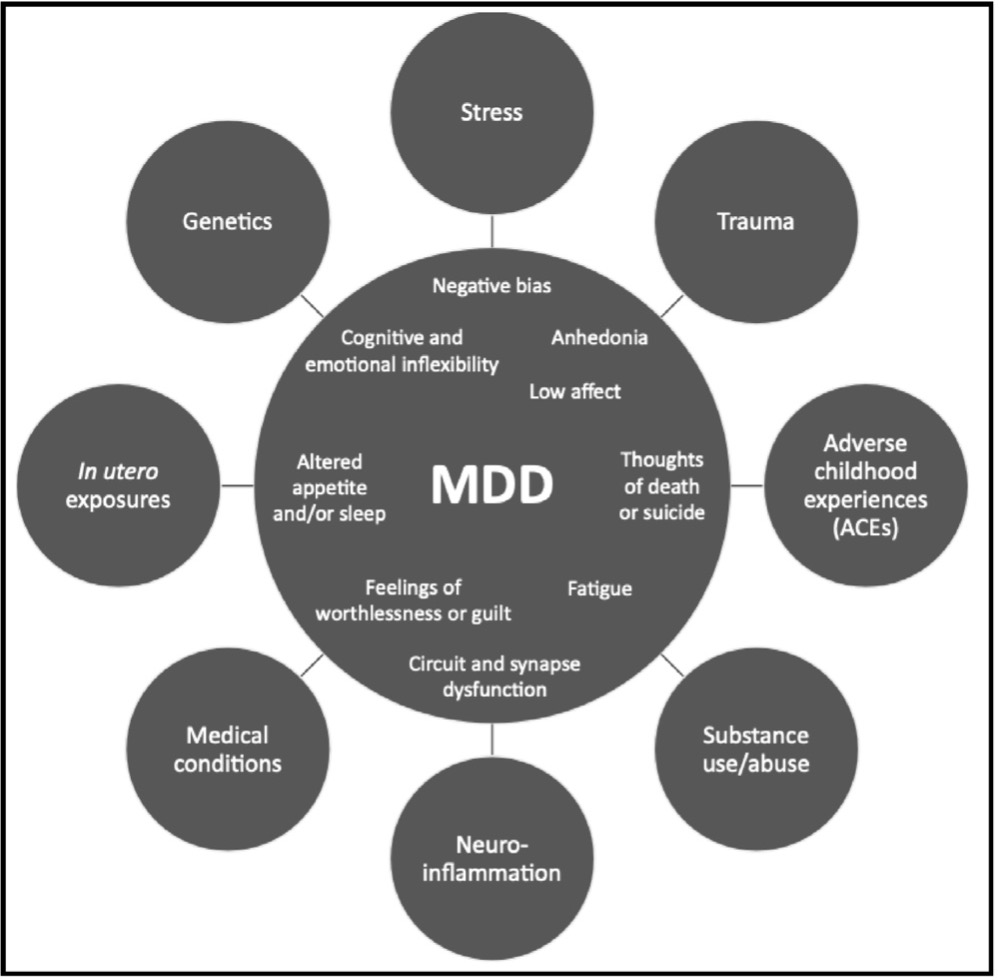
Figure 1. Multiple biopsychosocial factors can interact with each other and converge on MDD pathophysiology. MDD is a complex, multifactorial, biopsychosocial disorder with no single cause or homogenous presentation of symptoms. Factors such as stress, trauma, genetics, and more can contribute to MDD pathophysiology, which involves symptoms such as anhedonia, low affect, negativity bias, and cognitive and emotional inflexibility. Though the contributing factors and presentation of symptoms vary from patient to patient, MDD can be understood as inflexibility in circuits that process cognitive and emotional information and regulate motivation and arousal.
New hypotheses of MDD will include these factors: genetics (including epigenetics), stress, trauma, substance use and abuse, neuroinflammation, adverse childhood experiences (which seem to be a continuing story in our neoliberal world), and even in utero exposure to environmental insults. A simple chemical imbalance cannot account for what is known today. MDD can be understood as caused by inflexibility in neurocognitive processing. It follows that treatment for MDD should be understood as enhancing neuroplasticity:
A growing body of recent evidence suggests that treatments for MDD work…by enhancing neuroplasticity, rewiring dysfunctional brain circuits and synapses in adaptive ways that allow patients to become “unstuck” from negative thoughts, emotions, and behaviors. The broadest definition of neuroplasticity is “the brain’s ability to change”…neuroplasticity at the cellular and molecular level (involves) changes in the connections between neurons…At the network level…changes in the activity, connectivity, and/or volume of brain regions as large-scale indications that neuroplasticity has occurred. Evidence that neuroplasticity has taken place can also be seen through lasting changes in cognitive and emotional behaviors. Collectively, neuroplasticity represents a category of mechanisms that treat MDD by restoring synaptic strength and functional connectivity in dysfunctional brain circuits…both pharmacological and nonpharmacological treatments for MDD engage multiple mechanisms of neuroplasticity, which drives their therapeutic efficacy. These mechanisms of neuroplasticity underly the sustained effects of pharmacological and non-pharmacological treatments.
This explanation demands much more than a simple chemical imbalance. Therefore, although monoamines are still essential for antidepressant effects, they “likely play two major roles in facilitating the therapeutic response: (1) triggering downstream molecular cascades that result in neuroplasticity more chronically and (2) changing emotional processing and behavior more acutely.” In retrospect, various lines of evidence support this more expansive hypothesis, which also explains why “talk therapy” works for many patients. The latter is more expensive than a pill, however. Moreover, going beyond serotonergic therapy will be important for those MDD patients – children, teenagers, and young adults under the age of 25 (and their families) – who have a small but significantly increased risk of suicide when treated with conventional antidepressants.
Another problem with typical antidepressants is that their therapeutic effects are slow and variable. In contrast, recent studies have shown that a single subanesthetic dose of ketamine produces symptomatic relief of MDD symptoms within 24 hours of administration. Repeated doses of ketamine can be more efficacious in clinical trials than conventional antidepressants. The intranasal formulation of ketamine (Spravato®) is the first truly novel antidepressant in 50 years. It has been shown that ketamine induces neuroplasticity “in regions of the brain associated with reward, emotion, and cognitive function…and increasing excitation in hypoactive regions of the brain.” Perhaps Elon Musk is on to something?
So, treatments that raise serotonin levels help some with MDD, most likely by rewiring parts of the brain that regulate affect and sense of wellbeing. The chemical imbalance hypothesis was not so much wrong as simplistic, and Biomedicine has taken advantage of its opportunities. Other examples of anchoring include the overuse of statins, which are among the most prescribed drugs in the United States. Cardiovascular disease (CVD) is still the leading cause of death in these same United States after all these years. It is not that statins have no use in patients with severe CVD, but the notion that simple reduction in plasma cholesterol is indicated for most of us is the result of a rank oversimplification by Biomedicine of the relevant metabolism. Concept anchoring can persist for a very long time, especially in the world of Biomedicine.
Finally, the authors end with the following, which applies to all of Biomedical Science at all times and in all cases:
Conveying to a general audience the nuances and caveats inherent in a complex disorder and its treatment mechanisms may seem like a daunting task. However, as researchers and clinicians, we have a responsibility to explain to patients and the public how MDD and its treatments are currently conceptualized as simply as possible, yet without sacrificing accuracy. If we fail to do so, the risks are great: the void will inevitably be filled by unintentional or even intentional distortions that have the potential to increase misinformation and stigma while eroding public trust in science and medicine. In the end, communicating research is just as important as the research itself.
There can be nothing to add to this. It applies to every science.
A Short Note on Reading the Scientific Literature: This review on MDD is technically demanding but exemplary. One minor but important meta-point: The manuscript was submitted on 17 November 2023 and in revised form on 15 May 2024. It was accepted for publication on 21 May 2024 and published online on 31 May 2024. We hear a lot about this or that paper having been “peer reviewed.” Review and revision of this paper took six months of effort by the authors, reviewers, and editors. This indicates to me they all wanted to get it right. This time frame seems about right for something as important as treatments for MDD. “Slow and correct” is much better than “fast and whatever.”
Notes
(1) I will never forget the first time I watched a sales representative use a primitive version of image analysis to “prepare” an image for publication. My first thought: This will not end well. Digital manipulation allowed scientists to get sloppy with technique and then, for some, sloppy in other things. Good journals now scan illustrations for evidence of manipulation. Some are beginning to scan for evidence of AI, which will be more difficult to catch.
(2) Charles Lieber, the former chair of the Harvard Department of Chemistry and Chemical Biology, was convicted of lying about his professional relationship with Wuhan University of Technology and China’s Thousand Talents Program in a remarkable application of accountability (and filing false tax returns, naturally; why not tax evasion?). I am personally familiar with a case in which an academic scientist was found to have engaged in misconduct in grant applications submitted to NIH (~$8M) that resulted in no consequences for the institution, which should have been severe. To my knowledge, the miscreant (digital image manipulation, among other things, and clearly by this scientist and not someone else in the laboratory) subsequently taught science at a local high school, briefly, until his past became public knowledge due to the work of a good local newspaper.